Section 1.2: Chemical Bonds and Reactions
Introduction
Life depends on an intricate web of chemical reactions that assemble molecules, break them down, and transform them into usable forms of energy. At the heart of these processes are chemical bonds—the forces that hold atoms together—and the reactions in which these bonds are broken and reformed. Understanding bonds and reactions is essential for grasping how cells build macromolecules, harvest energy, and maintain homeostasis.
1. The Role of Chemical Bonds in Biology
Atoms are inherently reactive because they seek stability in their outermost electron shells. By forming bonds, atoms achieve more stable configurations, and these bonds determine the shape, function, and behavior of biological molecules.
- Bond strength and bond type dictate whether molecules are stable, reactive, or easily broken down.
- The rearrangement of bonds underlies all biochemical reactions, from DNA replication to muscle contraction.
2. Types of Chemical Bonds
2.1 Covalent Bonds – Sharing Electrons
A covalent bond forms when two atoms share pairs of electrons. These bonds are incredibly strong and stable under biological conditions, making them the primary force that holds together the backbones of large biological molecules like proteins and DNA.
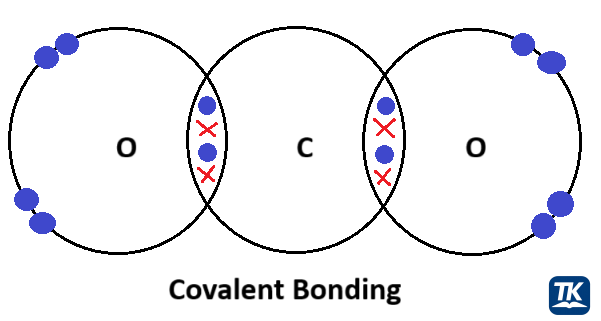
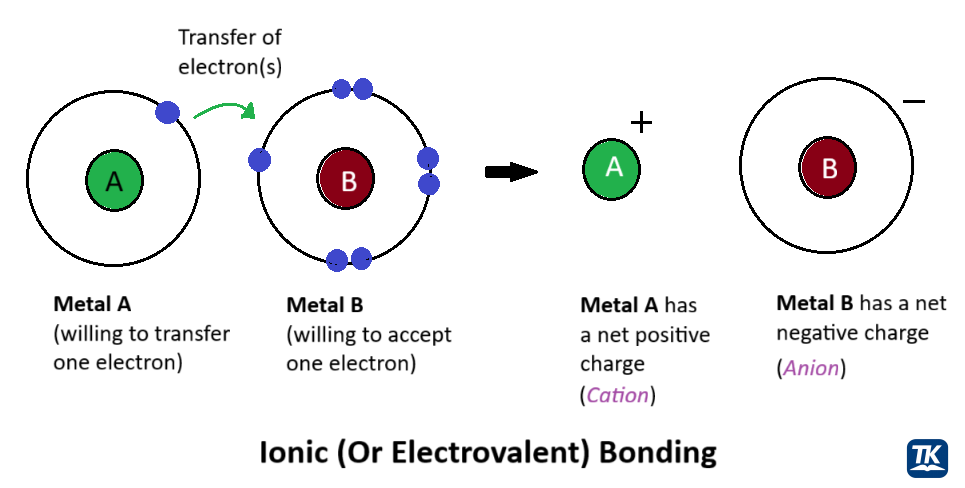
Figure 1.2.1: A comparison of covalent and ionic bonds. In a covalent bond, electrons are shared between atoms, while in an ionic bond, electrons are transferred, creating charged ions that are attracted to one another.
Polar vs. Nonpolar Covalent Bonds
- Nonpolar covalent bonds occur when electrons are shared equally between two atoms with similar electronegativity (e.g., the bond between two carbon atoms in a hydrocarbon chain). These bonds do not create partial charges.
- Polar covalent bonds form when electrons are shared unequally due to a difference in electronegativity. The more electronegative atom (like oxygen or nitrogen) will pull the shared electrons closer, creating a partial negative charge (δ⁻), while the other atom develops a partial positive charge (δ⁺). Water (H₂O) is a classic example of a molecule with polar covalent bonds.
This polarity is critical in biology as it gives rise to hydrogen bonding, solubility in water, and the separation of hydrophilic (water-loving) and hydrophobic (water-fearing) molecules, which is fundamental to cell membrane structure.
2.2 Ionic Bonds – Electron Transfer
An ionic bond results when one atom is so much more electronegative than another that it strips an electron completely away, forming charged ions. The resulting positive (cation) and negative (anion) ions are held together by electrostatic attraction.
Biological roles:
- While strong in a dry crystalline state, ionic bonds are much weaker in aqueous environments like the cytoplasm of a cell. This allows salts (like NaCl) to readily dissociate into ions (Na⁺ and Cl⁻), which are essential for processes like nerve transmission, muscle contraction, and fluid balance.
- In some cases, ionic bonds provide structural support, such as the calcium phosphate crystals that give strength to bones and teeth.
2.3 Hydrogen Bonds – Weak but Essential
A hydrogen bond is a weak, non-covalent attraction between a partially positive hydrogen atom (from a polar covalent bond) and a partially negative atom (like oxygen or nitrogen) in a neighboring molecule or part of the same molecule.
These bonds are individually weak but collectively very strong, making them dynamic and easily broken and reformed. This characteristic is crucial for many biological processes.
Biological roles:
- Holding together the two strands of the DNA double-helix.
- Maintaining the secondary and tertiary structures of proteins, which are critical for their function.
- Giving water its unique properties (high cohesion, surface tension, and heat capacity) that are essential for life.
2.4 Van der Waals Interactions
These are the weakest of all intermolecular attractions. Van der Waals interactions occur due to temporary, asymmetrical electron distributions that create fleeting positive and negative regions in molecules. These "hot spots" allow molecules to briefly attract one another.
Biological roles:
- Though individually weak, when many of these interactions occur simultaneously over large molecules, they can be quite strong. This is crucial for:
- The specific and precise binding of an enzyme to its substrate.
- The proper folding of proteins into their functional three-dimensional shapes.
- The stable packing of nonpolar molecules in cell membranes.
3. Chemical Reactions – Transforming Matter
A chemical reaction occurs when chemical bonds are broken and new ones are formed, leading to a rearrangement of atoms into new substances. The starting materials are called reactants, and the resulting substances are called products.
Example:
Cellular respiration:
C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + Energy (ATP)
Photosynthesis:
6CO₂ + 6H₂O + Light → C₆H₁₂O₆ + 6O₂
Reactions are often reversible, indicated by a double arrow (⇌), and their direction can be influenced by factors like the concentration of reactants and products.
3.1 Types of Chemical Reactions in Biology
- Synthesis (Anabolic) Reactions: Build larger molecules from smaller ones. These reactions require an input of energy and are essential for growth and repair. Example: Dehydration synthesis linking amino acids to form a protein.
- Decomposition (Catabolic) Reactions: Break down larger molecules into smaller ones. These reactions often release energy and are crucial for digestion and energy production. Example: Hydrolysis of starch into glucose molecules.
- Oxidation-Reduction (Redox) Reactions: Involve the transfer of electrons between molecules. Oxidation is the loss of electrons (or hydrogen), while Reduction is the gain of electrons (or hydrogen). These reactions are central to energy metabolism, such as cellular respiration and photosynthesis.
4. Energy in Chemical Reactions
All chemical reactions involve a change in energy. The energy stored in the chemical bonds of molecules is a form of potential energy. When bonds are broken and new ones form, energy is either absorbed or released.
4.1 Exergonic vs. Endergonic Reactions
- Exergonic reactions are reactions that release energy. The products have less potential energy than the reactants. The breakdown of glucose during cellular respiration is an example.
- Endergonic reactions are reactions that require a net input of energy. The products have more potential energy than the reactants. The synthesis of macromolecules is a prime example.
Biological systems efficiently couple these reactions: the energy released by an exergonic reaction (like ATP hydrolysis) is used to power an endergonic reaction (like muscle contraction).
5. Activation Energy and Catalysts
Even spontaneous, exergonic reactions require an initial input of energy to get started. This energy is called activation energy, and it is needed to destabilize existing bonds and allow new ones to form.
Without a mechanism to lower this energy barrier, life-sustaining reactions would be too slow to support life. This is where enzymes come in.
Enzymes are biological catalysts, typically proteins, that significantly lower the activation energy of a reaction. They do this by binding to specific reactants (substrates) at their active site and orienting them to facilitate bond-breaking and bond-forming. Enzymes are not consumed in the reaction and can be used over and over again.
6. Water and Chemical Reactions
Water is a critical component of virtually all biological reactions.
- Hydrolysis reactions use water to break down large polymers into their smaller monomer subunits. Water is split (hydro = water, lysis = break) and its atoms are added to the monomers.
- Dehydration synthesis reactions (also called condensation reactions) build polymers by linking monomers together. A water molecule is removed (dehydration) as a new covalent bond is formed.
7. Chemical Equilibrium in Biology
Most biological reactions are reversible. At chemical equilibrium, the forward and reverse reactions occur at equal rates, and the concentrations of reactants and products remain stable. However, cells rarely operate at true equilibrium. Instead, they constantly push reactions in a desired direction by:
- **Changing concentrations:** Continuously producing new reactants or removing products to prevent equilibrium from being reached.
- **Using enzymes:** Controlling the speed of forward and reverse reactions to maintain a dynamic steady state.
- **Coupling reactions:** Using the energy from an exergonic reaction to drive an endergonic reaction, effectively pulling the reaction forward.
For example, cellular respiration never truly reaches equilibrium because its products (CO₂, water, and ATP) are constantly used or removed, keeping the reaction moving forward to generate energy.
8. Biological Relevance of Bonds and Reactions
Understanding bonds and reactions is fundamental to all of biology. They are the basis for:
- Metabolism: The sum of all chemical reactions in an organism. These reactions are divided into two main categories: anabolism (building up, which requires energy) and catabolism (breaking down, which releases energy).
- Homeostasis: Chemical reactions, such as those involving buffers, maintain stable internal conditions (e.g., pH balance) essential for life.
- Genetic Information: The structure of DNA and RNA relies on strong covalent bonds for the sugar-phosphate backbone and weak hydrogen bonds for the base pairs. This dual-strength structure allows the genetic code to be stable while also being easily "unzipped" for replication and transcription.
- Proteins: The specific sequence of amino acids (linked by covalent peptide bonds) and the various bonding interactions (hydrogen, ionic, van der Waals) give proteins their complex 3D shapes, which are necessary for their diverse functions.
- Energy Transfer: The energy stored in the covalent bonds of molecules like ATP is the universal currency for powering cellular work. This energy is released and used through a series of chemical reactions.
Check Your Understanding
1. A scientist is studying a new molecule. They find that it is stable and nonpolar. Based on this information, which type of bond is most likely holding the atoms together?
a) Nonpolar covalent bonds because they involve equal sharing of electrons, leading to no charge separation.
2. Explain how hydrogen bonds are essential for both DNA and protein function, despite being relatively weak bonds.
b) Hydrogen bonds are essential because they are individually weak but collectively strong. This allows them to hold the two strands of DNA together and stabilize the 3D shape of proteins, while also being easily broken and reformed, which is necessary for processes like DNA replication and protein folding.
3. A metabolic pathway breaks down a complex carbohydrate into simple sugars and releases energy. Is this pathway anabolic or catabolic? Is it exergonic or endergonic?
c) This is a catabolic pathway because it breaks down a large molecule into smaller ones. It is also an exergonic reaction because it releases energy.
